
SARS-CoV-2 monitoring needs more than just antibodies...
Complete your view with T cells
T-SPOT® Discovery SARS-CoV-2: Proven T cell technology
The T-SPOT Discovery SARS-CoV-2 kit uses proven T cell technology to detect and measure the strength of the T cell response to SARS-CoV-2. We have over 17 years’ experience in developing leading T cell technology, and work in collaboration with government bodies including Public Health England and UK Vaccines Task Force.
Why choose the T-SPOT technology?
The T-SPOT technology is unique as the only globally regulated ELISPOT platform, currently being used clinically for identifying and measuring T cells. The platform makes highly standardised measurement of the T cell response easy through centralisation of fresh blood samples from different study locations, as well as enabling automation leading to ease of use and scalability. These attributes are essential in large-scale studies, or for more widespread testing.
Tested technology
18 years’ experience of clinical T cell measurement using the T-SPOT technology
> 20M tests sold
Experienced manufacturer of IVD tests and approved in over 50 countries
Central processing
Extended sample stability (54hr) and automation for flexible sample processing
Optimal antigens
Investigate the full breath of the T cell immune response
RESOURCE CENTRE
This is the central resource for all things T-SPOT Discovery SARS-CoV-2 related. Find out more about our leading T cell technology and further detailed information about how this technology can be applied to SARS-CoV-2.
How the T-SPOT technology works
Our T-SPOT test based on ELISPOT technology is normalised for both cell number and culture conditions. This means that the test standardises the number of cells and removes serum factors that could adversely affect results, making it the most sensitive and specific test for T cell measurement.
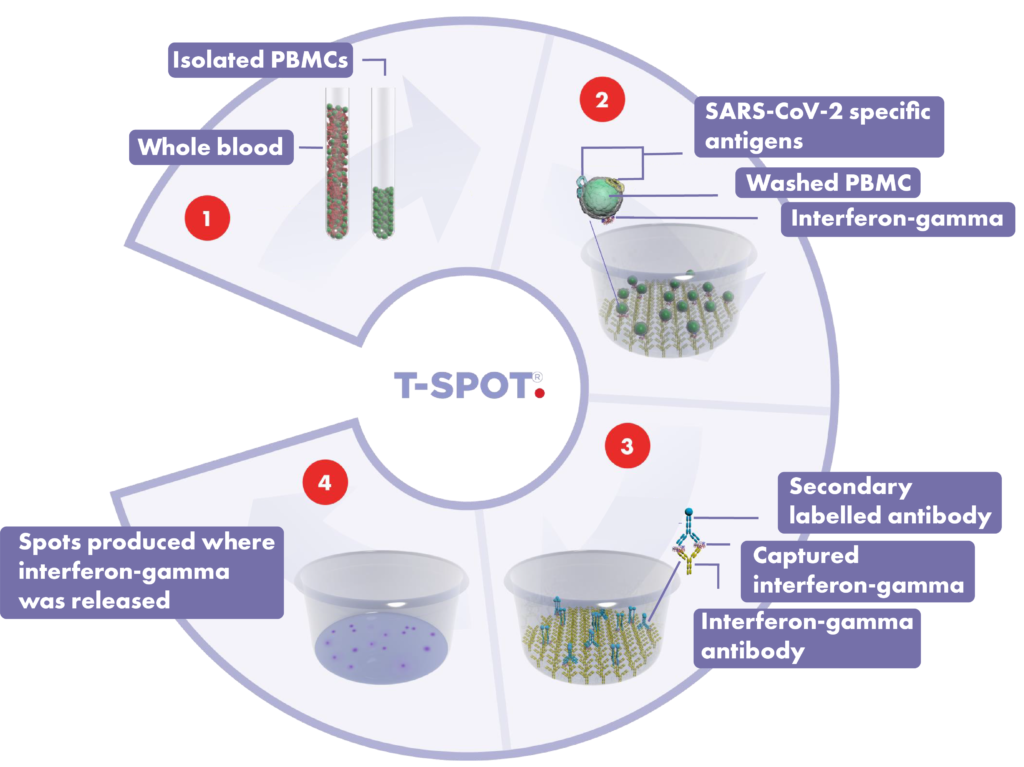
- A blood sample is collected using routine phlebotomy and a standard blood collection tube from which a subset of white blood cells, known as peripheral blood mononuclear cells (PBMCs), are isolated. The cells are washed, counted and normalised to create a standard cell suspension.
- A standard number of cells are added into specially designed plates and stimulated with antigens specific to the disease under study. Cells responding to these antigens release a chemical messenger known as a cytokine.
- Cytokine antibodies are used to directly capture the cytokine as it is released by the cells. A secondary labelled antibody is added and binds to the captured cytokine.
- A detection reagent is added and reacts with the secondary labelled antibody. This reaction produces spots, which are a footprint of where the cytokine was released. Spots are then enumerated.
Latest News
December 16, 2020
Oxford Immunotec Announces the Foundation of the Global T Cell Expert Consortium
